Organ Developmental Engineering
- Associate Professor
- ISOTANI Ayako

- Assistant Professor
- NAKAGAWA Tatsuya

- Labs HP
- https://bsw3.naist.jp/isotani/index-en.html
Outline of Research and Education
In mammals, until the eight-cell embryo stage, fertilized eggs have totipotency, meaning that each cell can differentiate into all kinds of cell. In blastocyst-stage embryos just before implantation, the cells’ fates are divided into the trophectoderm (TE), which will develop into placental tissue, and the inner cell mass (ICM), which has pluripotency in that its cells will develop into three germ layers, including germline cells. Embryonic stem cells (ESCs) were established from ICM, promoting the study of regenerative medicine and led to the discovery of induced pluripotent stem cells (iPSCs). We combine these early embryos, ESCs/iPSCs, and developmental technology with the aim of performing basic studies that will lead to regenerative medicine using animal models.
Major Research Topics
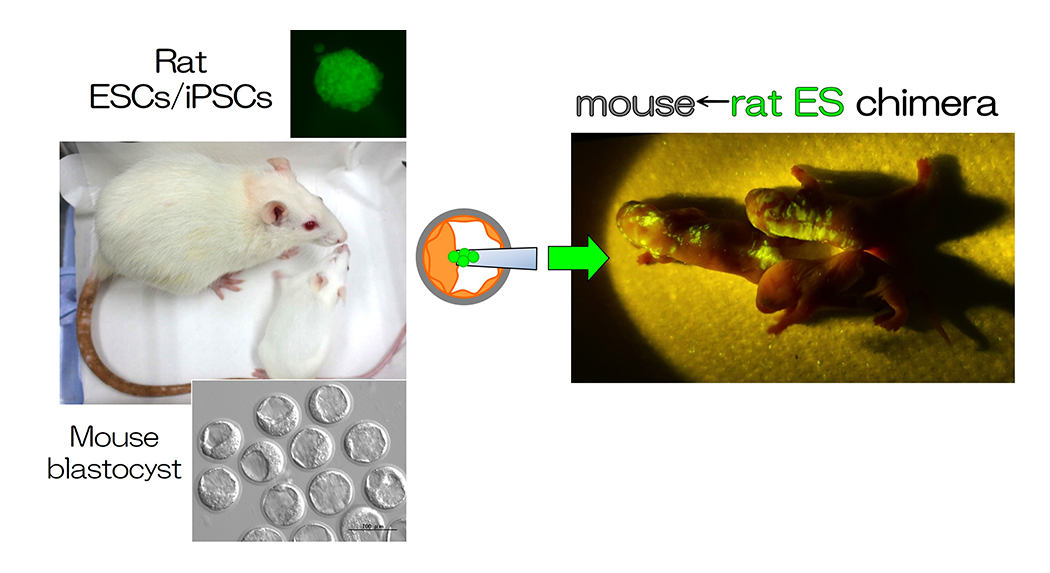
Model of organ formation using interspecies chimeras
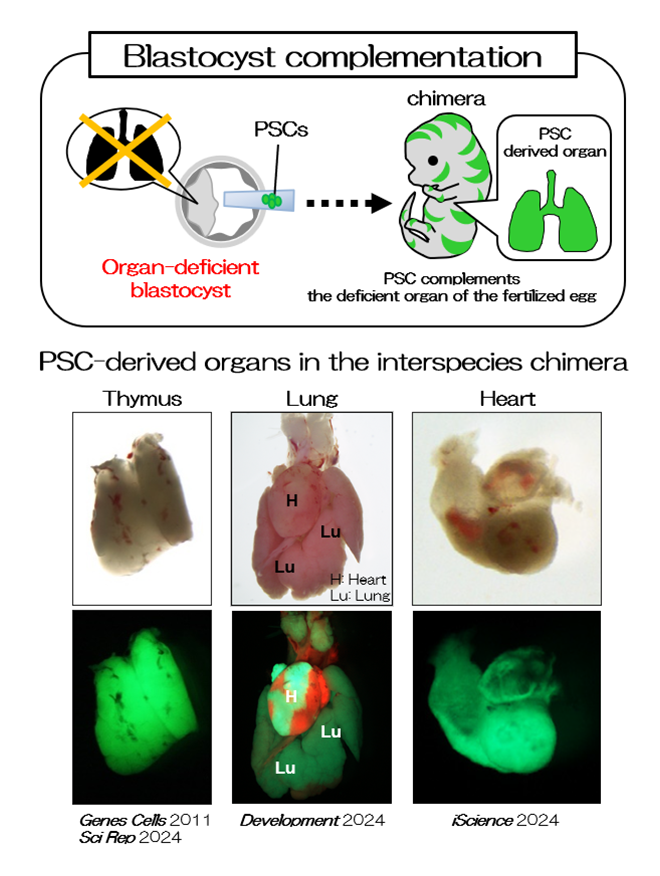
Interspecies chimeras containing both mouse and rat cells were generated using blastocysts and ESCs (Fig. 1 and 2, REF 1-3). When we injected rat ES cells into blastocysts of organ-deficient mice, we could produce a rat organ in chimeric animals. This indicates the formation of an organ from ES cells in interspecies conditions (Fig. 2, REF 1-3). Moreover, we could detect rat spermatozoa in mouse←rat ES chimeric testes. Rat pups were generated from rat spermatozoa in the interspecies chimeric testes by intracytoplasmic injections, and the normal germline potential of rat spermatozoa in the interspecies chimeric testes was demonstrated. Findings on the functions of organs, tissues, and cells developed in interspecies chimeras are valuable for future translational research.
Trials of novel animal models
Gene knockout animals can easily be generated using genome editing systems such as the CRISPR/Cas9 system. Complicated gene modification can be performed using the combination of this system and ESCs. However, ESC has no potency to differentiate into the placental lineage. The tetraploid embryo, which is produced by fusing two blastomeres at the 2-cell stage, can contribute to placental lineage only, not fetus tissues. In the tetraploid complementation method, whose way is gene-modified ESCs injected into the tetraploid embryo, the fetus or pups are derived from ESCs only, and then it is possible to be F0 analysis (Fig. 3, REF 3, 5 and 6).
We aim to produce novel animal models using these technologies.
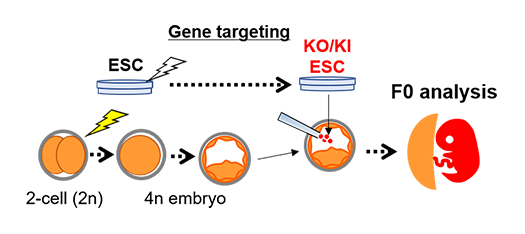
When the gene-modified ESCs are injected into the tetraploid embryo, fetus or pups consist of ESC-derived cells only. We can directly analyze F0 fetuses or pups, not through the next generation.

 NAIST Edge BIO(
NAIST Edge BIO(