Plant Growth Regulation
- Professor
- UMEDA Masaaki

- Assistant Professor
- Zhang Ye

- KAWAMOTO Nozomi

- Labs HP
- https://bsw3.naist.jp/umeda/
Outline of Research and Education
Plants continuously produce organs throughout their life. This feature renders them distinct from animals, in which organ formation ceases soon after embryogenesis. We aim to understand the mechanisms of tissue maintenance and regeneration, stress response and DNA polyploidization that support sustained plant growth under changing environments. Our study will contribute to the development of technologies to increase plant biomass and food production, and to protect global plant resources.
Major Research Topics
Tissue maintenance and regeneration mechanisms in plants
Plants possess a remarkable ability for organogenesis and tissue regeneration following injury. However, unlike animals, they do not develop cancer. Our research focuses on the genome maintenance mechanisms that contribute to plant longevity. Additionally, we aim to understand the molecular basis of tissue maintenance and regeneration by investigating how neighboring cells initiate regenerative division after cell death and how autonomous mechanisms suppress overproliferation in tissues. Our study will reveal how plants can continuously and accurately generate organs throughout their lifetime without a central nervous system.
Plant growth regulation in response to abiotic stress
Plant growth is usually inhibited under stressful conditions because plants need to use energy for coping with stress, rather than for organ growth. We have recently identified the signaling cascade that triggers G2 cell cycle arrest in response to multiple stresses, thereby functioning as a module for stress-induced growth arrest. We are studying how this cascade orchestrates the expression of G2/M-specific genes and whether it is conserved among plant species. We are also generating multiple stress-tolerant crops, which can continuously grow under changing environments, by modifying the signaling components.
Mechanisms for induction of DNA polyploidization
In many plant species, cells start DNA polyploidization, called endoreplication, after the cessation of cell division. Endoreplication promotes enlargement of individual cells and organs; thus, it greatly contributes to plant biomass production. While previous studies demonstrated that inhibition of cell cycle progression induces the onset of endoreplication, we recently found that chromatin decondensation can be another cause that triggers endoreplication. We are focusing on the key epigenetic factors controlling endoreplication and investigating the involvement of chromatin dynamics in the transition from cell division to DNA polyploidization. We are also developing technologies to enhance DNA polyploidization in crops and woody plants, aiming to increase food and biomass production.

Stem cell death occurs when Arabidopsis roots are exposed to DNA damage. Subsequently, cell division is activated in neighboring cells, facilitating tissue regeneration. Green cells trigger cell division next to the dead cells.
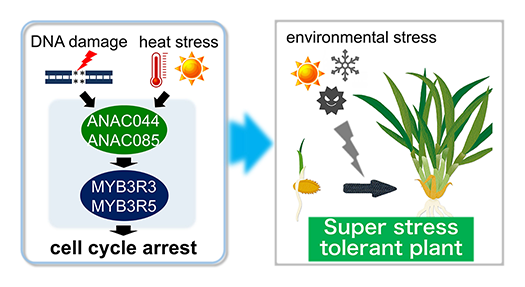
Transcription factors MYB3R3/5 cause G2 arrest in response to DNA damage and heat stress. Suppression of this module will enable us to generate super stress tolerant plants.
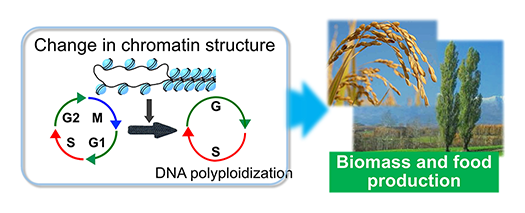
Change in chromatin structure and cell cycle retardation induce DNA polyploidization. Our goal is to develop technologies that enhance food and biomass production while contributing to carbon recycling.
References
- Takahashi N. et al., J. Exp. Bot., 75, 1364-1375, 2024
- Takatsuka H. et al., J. Exp. Bot., 74, 3579-3594, 2023
- Takahashi N. et al., Sci. Adv., 7, eabg0993, 2021
- Shimotohno A. et al., Annu. Rev. Plant Biol., 72, 273-296, 2021
- Umeda M. et al., Plant J., 106, 326-335, 2021
- Watanabe S. et al., Proc. Natl. Acad. Sci. USA, 117, 31500-31509, 2020
- Umeda M. et al., Curr. Opin. Plant Biol., 51, 1-6, 2019
- Takahashi N. et al., eLife, 8, e43944, 2019
- Takatsuka H. et al., Plant Physiol., 178, 1130-1141, 2018
- Ogita N. et al., Plant J., 94, 439-453, 2018
- Chen P. et al., Nature Commun., 8, 635, 2017
- Kobayashi K. et al., EMBO J., 34, 1992-2007, 2015
- Yin K. et al., Plant J., 80, 541-552, 2014
- Takahashi N. et al., Curr. Biol., 23, 1812-1817, 2013
- Yoshiyama K.O. et al., EMBO Rep., 14, 817-822, 2013

 NAIST Edge BIO(
NAIST Edge BIO(