Background
What Is a Parasitic Plant?

Fig1. Parasitic plants
From left to right; Striga hermonthica, Orobanche minor and Viscum album.
Parasitic plants have haustoria, specialized invasive organs that penetrate and absorb nutrient from the host tissue. There are an estimated 4500 species of parasitic plants, accounting for approximately 1% of all angiosperms. Parasitic plants can generally be described as facultative, obligate, hemi-, or holoparasitic, depending on their host dependency. Facultative parasites can survive without the host plant, whereas obligate parasites require nutrients from the host for their survival. Facultative parasites appear very similar to non-parasitic plants and have green photosynthetic leaves. By contrast, some obligate parasites have lost their photosynthetic capability as well as typical roots or leaves, often exhibiting a peculiar appearance. Parasites that have photosynthetic capabilities are termed hemiparasitic, and those plants that lack them are termed holoparasitic. Parasitic plants that attach to the stem of the host plant are stem parasites, and those that attach to the host’s root are root parasites.
Orobanchaceae
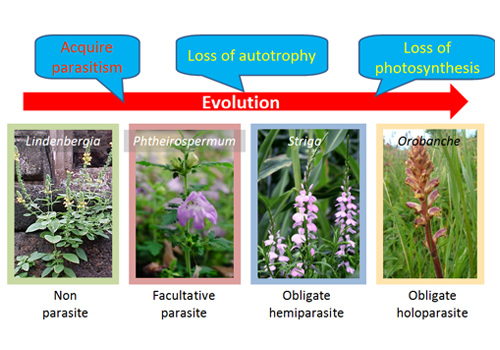
Fig2. Evolution of Orobanchaceae
Parasitic plants experienced several evolutional events to have variety in their parasitism.
Parasitic species arose via 11 or 12 independent evolutionary events over the course of angiosperm evolution. Orobanchaceae contains the largest number of root parasite species. All plants belonging to this family are parasitic, except for those of the genus Lindenbergia. This family is regarded as an ideal candidate for plant evolution research because it contains a wide range of facultative, obligate hemiparasitic, and obligate holoparasitic species.
Striga, Orobanche, and other obligate parasitic genera of family Orobanchaceae cause damage to their host crop plants, causing drastic yield losses worldwide. However, eradication of these weeds is difficult due to their high reproductive capacity and extremely small seeds. Their seeds germinate responding to strigolactone released from the host plants. Strigolactone also plays a vital role in plant growth and activation of mutualistic mycorrhizal fungi. Given that facultative parasitic plants of the family Orobanchaceae do not require strigolactone stimulation for seed germination, strigolactone stimulation is a feature acquired during evolution from facultative to obligate parasitism.
We pursue research aimed at elucidating the molecular mechanisms of parasitism using Striga (obligate parasite) and Phtheirospermum japonicum (facultative parasite), with the ultimate goal of solving agricultural problems arising from parasitic weeds.
Research Topics
1) Mechanisms of Haustorium Development
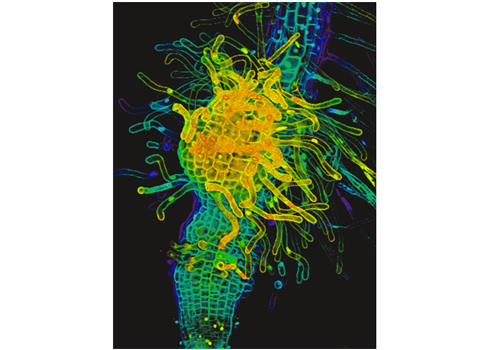
Fig3. Haustorium of P. japonicum.
Three-dimensional reconstructed haustorium formed on a P. japonicum root. Colors reflect the depth of focus plane.
When the root of a parasitic plant makes contact with the host plant root, the parasitic plant develops a haustorium, which penetrates into the host vascular system to absorb water and nutrients (Movie 1, Figure 2). The haustorium is an organ unique to parasitic plants. Via active cell division and differentiation, obligate parasites develop terminal haustoria, whereas facultative parasites develop lateral haustoria.
We generated mutagenized Phtheirospermum lines and screened mutants that exhibit defects in the haustorium formation. Through next-generation sequencing of the mutant genomes, we developed the methods to identify the genes responsible for the mutations. In addition, we conducted transcriptome analyses for Striga and P. japonicum, and identified genes upregulated during haustorium formation. Using reverse genetic approaches, we are working to characterize the gene function in parasitism.
2) Molecules That Induce Parasite Haustorium Formation
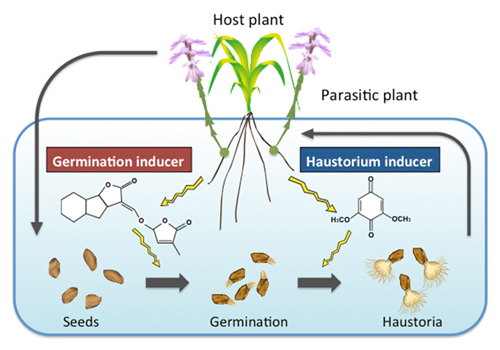
Fig4. Chemical communication between hosts and parasites.
The germination of Striga are provoked by host-derived strigoractone. Haustorium formation is also stimulated by host derived haustorium inducing factors.
Parasitic plants recognize small molecules released from the host plants in order to develop parasitic relationship. For example, Striga seed germination takes place in the presence of the plant hormone strigolactone. Haustorium formation is also activated by small molecules; 2,6-dimethoxy-p-benzoquinone(DMBQ) is identified as a host-derived haustorium inducer. However, little is known about how DMBQ is produced or delivered to its site of action. Molecules other than DMBQ also can induce haustorium formation. Using chemical and molecular genetics approaches, we will investigate the chemical features and mode of reaction of the molecules that mediate haustorium formation.
3) Evolution of Parasite Genome Architecture
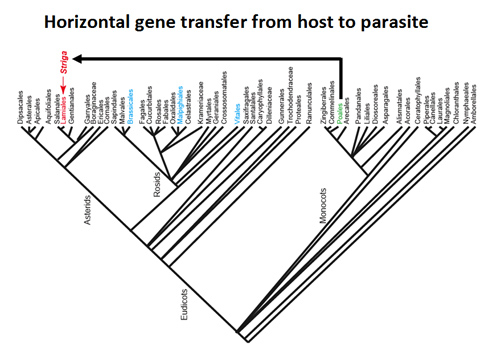
Fig5. Horizontal gene transfer from a host to a parasite.
Parasitic plant Striga acquires genes from their Poaceae hosts by horizontal gene transfer.
Recent advances in next-generation sequencing have accelerated whole-genome characterization of plant species. We determined the whole-genome sequences of Striga and P. japonicum. Our findings showed that these parasitic plants capture host genes via horizontal gene transfer. Using comparative genomics, we are investigating how the parasitic genome evolved to acquire parasitism and become diversified.





