Research
Xenogeneic Chimeras
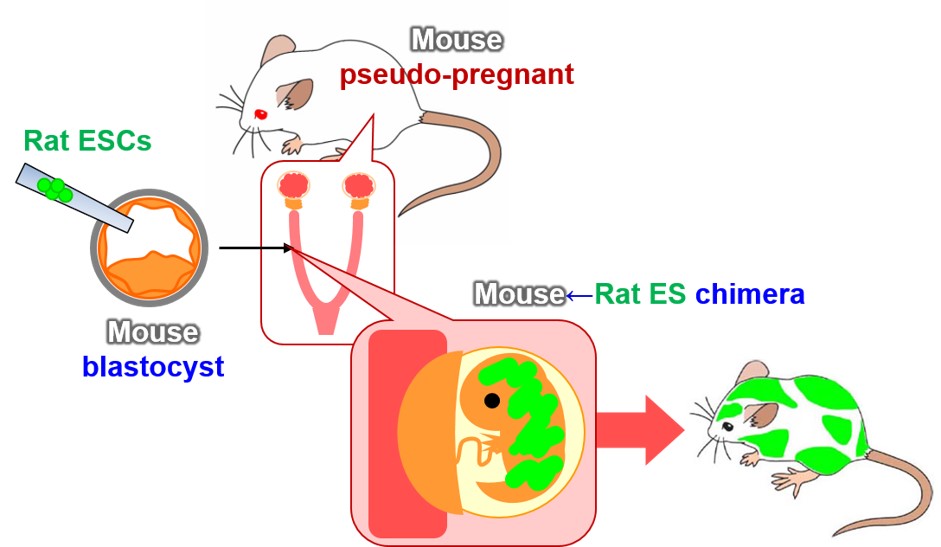
Despite multiple attempts in the 1970s to create various types of xenogeneic chimeras, the generation of a mouse↔rat chimera was not successful. In those days, chimeras were produced by the aggregation method, resulting in all of their organs and tissues, including the placenta, being composed of cells of heterogeneous origin. The failure was probably attributable to the maternal immune system, which detected and eliminated xenogeneic placental cells as foreign matter. This hypothesis is strongly supported by the successful generation of xenogeneic chimeras by injection of ES cells, which can differentiate into all types of tissues except for placenta (Figure 1, Ref. 1).
Chimeric Models of Organogenesis
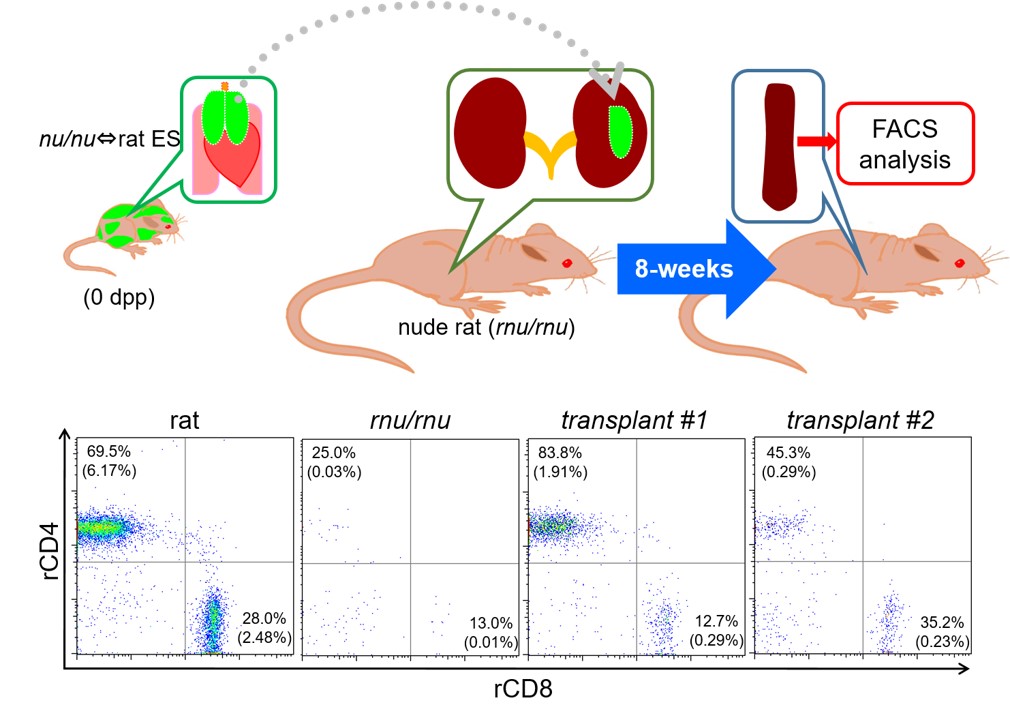
We produced xenogeneic chimeras by injecting ES cells of rat origin into blastocysts obtained from thymus-deprived nude mice. In those chimeric mice, the thymus consisted of rat-derived cells. Our findings suggested that interspecies chimeric models represent promising platforms for generating complex organs from ES or induced pluripotent stem (iPS) cells of the target species (Ref. 1). A thymus of rat ES origin formed in a xenogeneic chimera could effectively induce T-cell maturation (Figure 2). However, the thymus in the chimeras was considerably smaller than normal murine thymus. In addition, it remains to be seen whether T cells educated by the chimera’s thymus can effectively discriminate between self and non-self.
A future challenge for regenerative medicine is to determine whether the organs, tissues, and cells of xenogeneic chimeras function as efficiently as those of wild-type organisms. To investigate this issue, we will create xenogeneic models of organogenesis and use them to elucidate the factors necessary for normal biological functioning.
More New Experimental Models
The clustered regularly interspersed short palindromic repeat (CRISPR) technology combined with the use of Cas9 nucleases provides a next-generation technique for genome editing. Such genome-editing technology has enabled the creation of gene-knockout animals (Refs 2,3). When combined with ES or iPS cells, it allows for more complicate genome editing (Ref 4).
We created a mouse←rat chimera by injecting rat ES cells into mouse embryos. The testes of this chimera contained both mouse and rat sperm. The rat spermatozoa had the potential to fertilize rat oocytes, which produced live pups. In addition, we successfully produced genetically engineered rats using chimera-derived rat spermatozoa (Ref 5).
Leveraging genome-editing techniques, chimeric animal technologies, and experimental models of human disorders, as well as genetic engineering, cell engineering, and reproductive engineering methodologies, we continue to develop new experimental models that can help achieve breakthroughs in life science research.