Research outcomes
Miracle math determines the dynamically coordinated regulation of edge velocity by Rho GTPases
Miracle math determines the dynamically coordinated regulation of edge velocity by Rho GTPases
Mathematical model and motion-triggered average algorithm decode the coordinated regulation of cell-edge velocity by Rho GTPases.
Rho GTPases have a crucial role in the orchestration of cell movements. Cells use Rho GTPases to coordinate cytoskeletal reorganization in dynamically changing environments. Among these RhoGTPases, Cdc42 and Rac1 promote cytoskeletal formation, whereas RhoA is involved in myosin II-mediated cytoskeletal retraction and formation.
Simultaneous live observations of multiple GTPase activities and cell morphology changes by specific biosensors and the resulting spatiotemporal data might help to determine the coordinated regulation of morphological changes. However, various limitations in observing Rho GTPases simultaneously have caused difficulties in quantitatively assessing the dynamic, coordinated regulation of cell-edge movement by them.
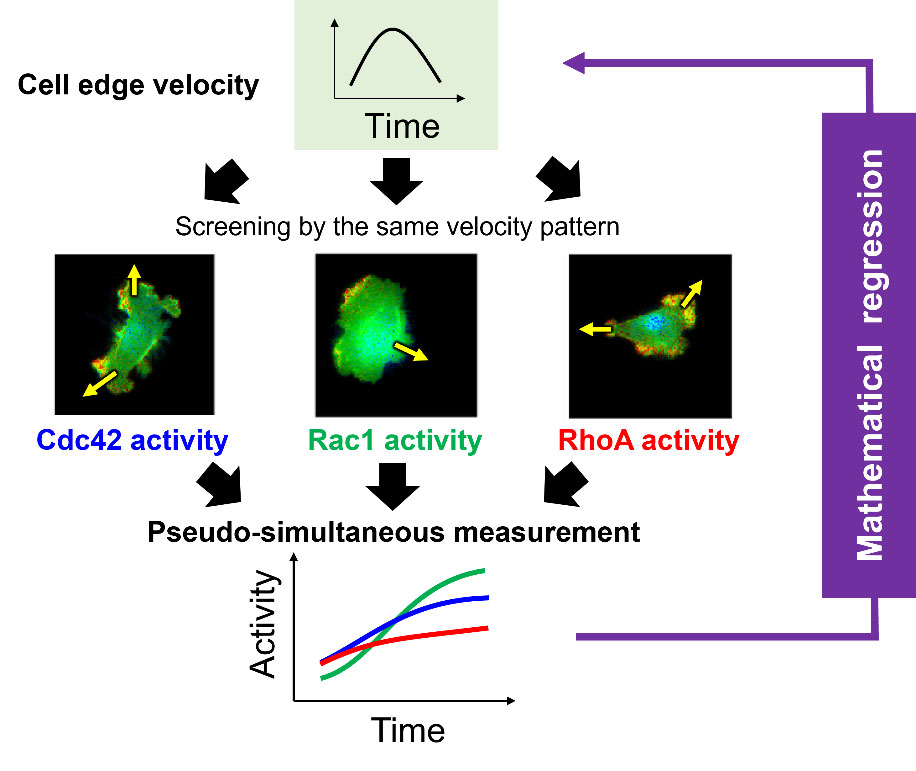
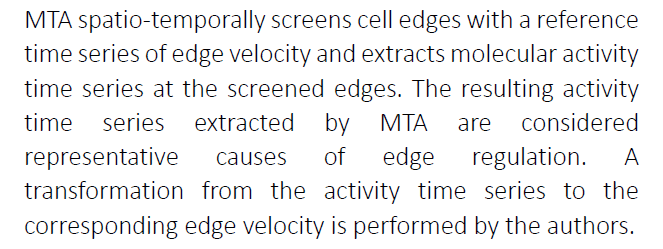
A new study, led by researchers from the Nara Institute of Science and Technology (NAIST), Japan, has developed the motion-triggered average (MTA), an algorithm that converts individual Rho GTPase observations into pseudo-simultaneous ones. The MTA screens and averages the molecular activity time series coinciding with the predetermined reference velocity time series of the cell edge.
MTA activities are consistent with known individual features and demonstrate interesting attributes, with the MTA activity time series highlighting the specific characteristics of each Rho GTPase clearly. Edge velocity is associated with differences among the activities of the Rho GTPases. This study has shown that during both expansion and retraction, molecular activities peaked later than velocities. Also, when the activity time series was shifted into the past, the temporal cross-correlation between one of these activities and velocity was maximal, consistent with previous studies.
The authors also propose a mathematical model to decode cell-edge velocity from the time series obtained through MTA, thereby providing evidence for the coordinated regulation of cell-edge velocity by Rho GTPases. They expressed the edge velocity as a function of Rho GTPase activities, thus taking into consideration the elastic properties of the cell membrane.
"The model offers a decoding method and numerical proof for the dynamically coordinated regulation of edge velocity by the three Rho GTPases," says Yuichi Sakumura.
The mathematical regression model used in this research predicts the edge velocity from the activities of the three Rho GTPases. The unknown edge velocity is predicted accurately, and the model provides numerical evidence that these Rho GTPases regulate edge movement. To examine the accuracy of this model's predictions, the errors of the time-series predictions were compared using 5-fold cross-validation. As the training data did not contain any time series of testing data, so these data were unknown to the model. However, the model could predict the velocities from activities accurately. The accurate velocity decodings showed that the regression equation approximates the general rule and that each of the 3 Rho GTPase activities represent partially the information that regulates edge velocity co-ordinatedly.
Data pre-processing using MTA combined with mathematical regression provides an effective strategy for reusing numerous individual observations of molecular activities. The key to successful analysis with MTA and regression lies in the number of time-series samples and the validity of the regression equation.
The combination of MTA and regression might be useful for time-series analysis of black-box systems. For any physical quantity, MTA can be applied to extract the average time series that triggers the time series of any phenotype by reusing a considerable amount of data obtained previously. Furthermore, analysis with MTA using the migratory cells of other species might yield different activity time series and new findings.
"Although the regression modeling in this study does not describe the detailed process, it serves not only to reveal informative factors, but also to make the black box a little less opaque," says Yuichi Sakumura.
MTA only extracts simultaneous time series of several different physical quantities; therefore, appropriate mathematical analysis methods are required to identify unknown causal relationships or feedbacks. Conversions between physical quantities must be fundamentally data-driven and empirical, just like the laws of physics. This study is significant because it decodes the velocities of a physical quantity other than biochemical signals. This study is also important because regression was performed using multiple factors.
###
Resource
・Title: Decoding cellular deformation from pseudo-simultaneously observed Rho GTPase activities
・Authors: Katsuyuki Kunida, Nobuhiro Takagi, Kazuhiro Aoki, Kazushi Ikeda, Takeshi Nakamura, Yuichi Sakumura
・Journal: Cell Reports
・DOI: 10.1016/j.celrep.2023.112071
・Information about the Data-driven Biology Laboratory can be found at the following website: https://bsw3.naist.jp/eng/courses/courses311.html
( April 07, 2023 )
