Research outcomes
Clarifying the Mechanism of Protein Insertion into the Cytoplasmic Membrane
Key points
- Determining the crystal structure of YidC, a membrane protein insertase that inserts proteins into the cytoplasmic membrane, for the first time in the world
- Clarifying details of the molecular mechanism of protein insertion into the plasma membrane, phenomena common to organisms from bacteria to humans
- Contributing to the advancement of life sciences research and laying the foundations for the development of novel drugs
Cells, the basic unit of all living organisms, are enclosed by the cytoplasmic membrane. Learning more about the functions of the cytoplasmic membrane is therefore essential for understanding basic life phenomena that are common to organisms from bacteria to humans. The cytoplasmic membrane contains the membrane protein insertase YidC, a vital factor that inserts proteins into the cytoplasmic membrane. However, the detailed structure of YidC has not yet been clarified and the molecular mechanism of YidC-mediated protein insertion into the cytoplasmic membrane remains unclear. The crystal structure of YidC was determined for the first time in the world by a research group led by Osamu Nureki (professor), Ryuichiro Ishitani (associate professor), and Kaoru Kumazaki (doctoral student) of the Graduate School of Science, The University of Tokyo; Shinobu Chiba (associate professor) of the Faculty of Life Sciences, Kyoto Sangyo University; and Tomoya Tsukazaki (associate professor) of the Graduate School of Biological Sciences, Nara Institute of Science and Technology. Further advancing their research on the basis of detailed structural information, they discovered an unexpected and unique structure of YidC that forms a hydrophilic (compatible with water) groove within a hydrophobic (incompatible with water) lipid bilayer. Also, they confirmed that the proteins inserted into the cytoplasmic membrane bind to the hydrophilic groove of YidC; thus, they proposed a new model of the molecular mechanism of YidC-mediated protein insertion into the cytoplasmic membrane. Their achievements of clarifying basic life phenomena common to all organisms will greatly contribute to the advancement of life sciences research. They will also lay the foundations for the development of novel antibiotics that target YidC in pathogenic bacteria because YidC is an essential protein for the growth of bacteria.
Their achievements were published online in the British scientific journal Nature on 17 April 2014..
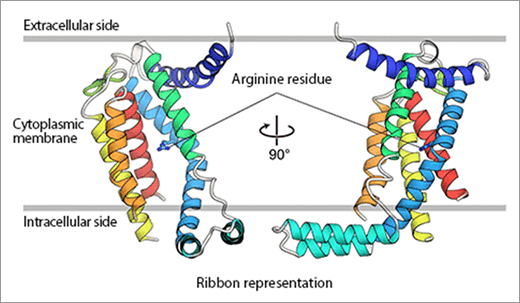
Fig. 1. The structure of YidC is represented by ribbon models.
The main part of YidC protein consists of five transmembrane α-helices (represented as ribbons), most of which are embedded within the cytoplasmic membrane. Arginine residues that are important for the activity of YidC are shown in the figure.
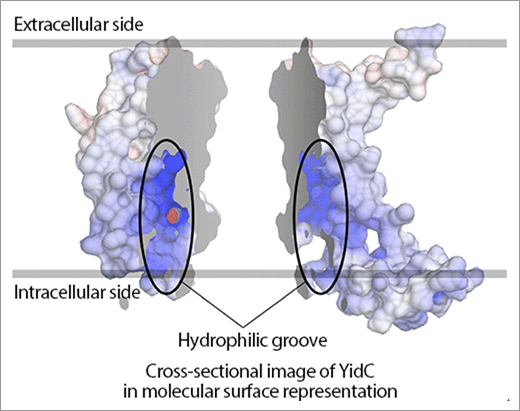
Fig. 2. Molecular surface representation of YidC.
Positive and negative charges are indicated in blue and red, respectively, in the molecular surface model of YidC. YidC is represented in two separate parts for ease of understanding. The model shows that YidC has a positively charged hydrophilic groove.
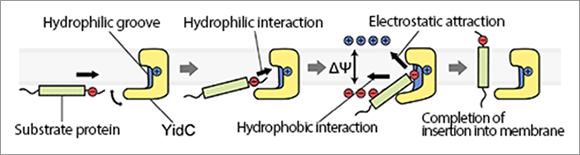
Fig. 3. Model of YidC-mediated protein insertion into membrane.
First, the negatively charged extracellular region of the substrate protein interacts with the positively charged hydrophilic groove of YidC. Then, protein insertion into the membrane is promoted by the hydrophobic interaction and the electrostatic attraction due to the membrane potential (the difference in electrical potential across the membrane). Finally, the substrate protein is released from YidC and completely inserted into the cytoplasmic membrane.
Publication
"Structural basis of Sec-independent membrane protein insertion by YidC"
Kaoru Kumazaki, Shinobu Chiba, Mizuki Takemoto, Arata Furukawa, Ken-ichi Nishiyama, Yasunori Sugano, Takaharu Mori, Naoshi Dohmae, Kunio Hirata, Yoshiko Nakada-Nakura, Andres D. Maturana, Yoshiki Tanaka, Hiroyuki Mori, Yuji Sugita, Fumio Arisaka, Koreaki Ito, Ryuichiro Ishitani, Tomoya Tsukazaki & Osamu Nureki
( February 13, 2015 )
