2025.10.02
Epigenetic Regulation of Neural Stem Cell Aging in the Mouse Hippocampus by Setd8 Downregulation
Neural Regeneration and Brain Repair ・ Postdoc ・ Kanae Matsuda
In our brain, there is a region called the hippocampus that governs higher functions such as memory and learning. Within the hippocampus, there exist “neural stem cells” that generate new neurons. It is known that the number and activity of neural stem cells decline with age, which is thought to contribute to the deterioration of memory and learning ability. However, the molecular mechanisms underlying these changes have not been fully elucidated. In particular, how gene expression in neural stem cells changes with aging, and how this is linked to epigenomic regulation (such as DNA methylation and histone modifications), has remained largely unclear.
To address this, our research group initiated a study with the aim of comprehensively elucidating the functional changes of neural stem cells during aging and ultimately contributing to the development of technologies for “cellular rejuvenation” and novel therapeutic approaches for age-related diseases. In this article, we introduce an overview of these research findings.
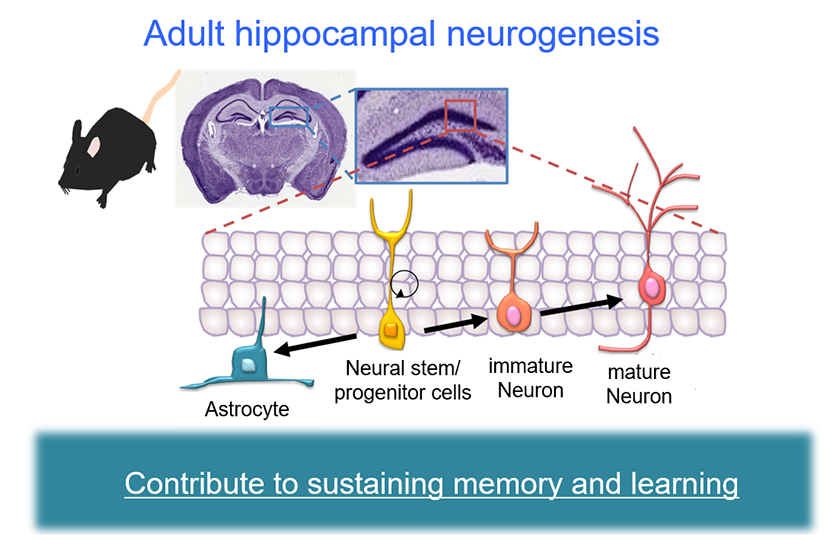
Figure1: Adult hippocampal neurogenesis.
In the hippocampus of the adult brain, including humans, neurons are generated from neural stem cells.
The newly generated neurons integrate into hippocampal neural circuits and contribute to the maintenance of memory and learning.
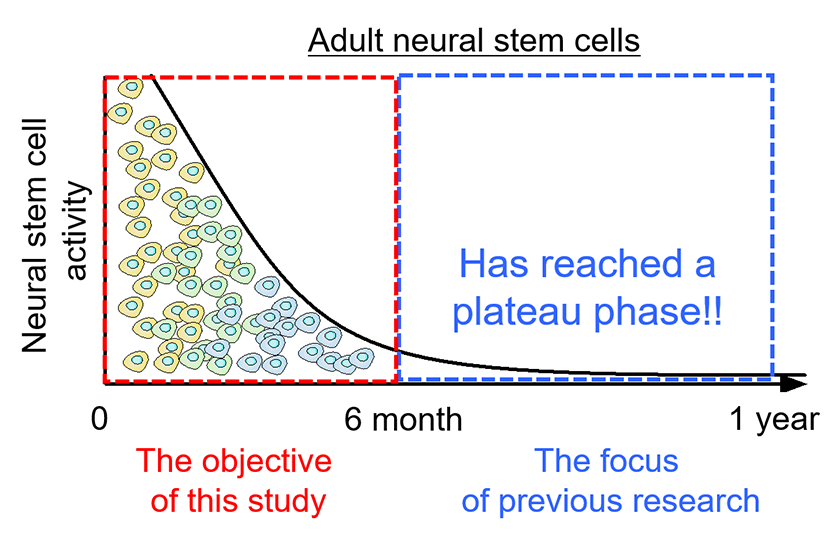
Figure2: Age-dependent decline of neural stem cell function.
With aging, the proliferative capacity and neurogenic potential of hippocampal neural stem cells in mice are rapidly lost. As a result, this decline is thought to contribute to future deterioration of cognitive function. Impairment of neural stem cell function has also been suggested to be associated with cognitive decline in Alzheimer’s disease.
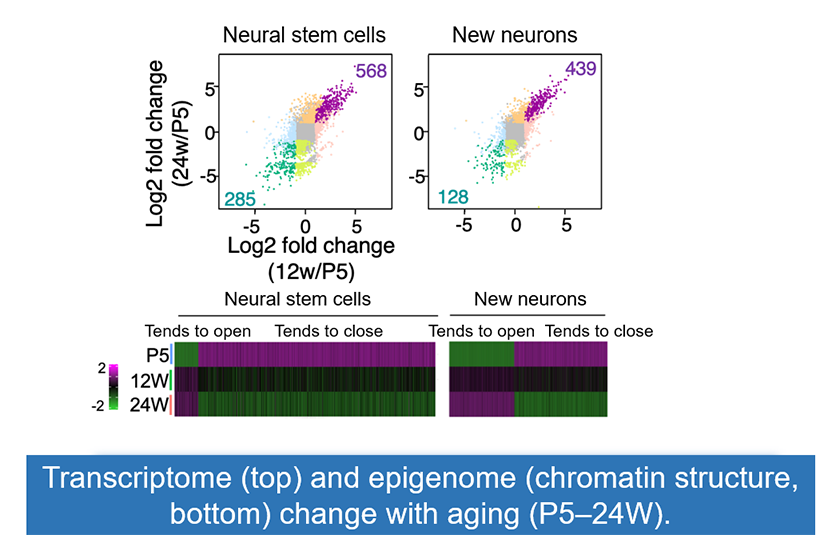
Figure3: Single-cell analysis of age-related changes in gene expression and the epigenome.
Using single-cell analysis, we performed a time-series assessment of gene expression (top) and epigenomic changes (bottom) in neural stem cells and their differentiation lineages. We found that hippocampal neural stem cells in adult mice already display aging-like alterations from a young age.
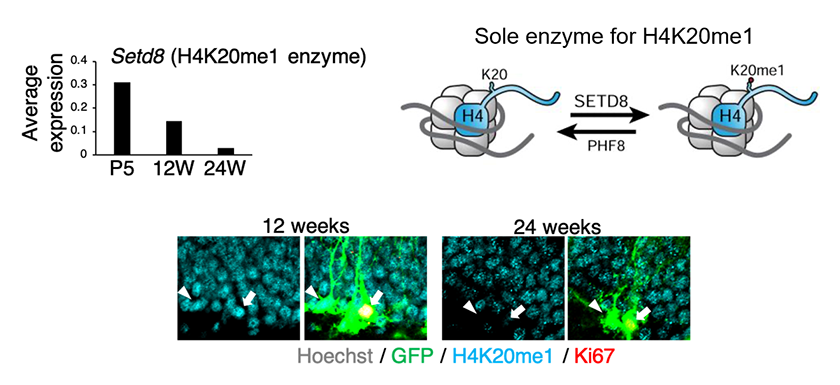
Figure4: Age-related decline in Setd8 expression (H4K20 monomethyl transferase)
Using single-cell analysis data, we found that in hippocampal neural stem cells of mice, Setd8 expression decreases with aging, resulting in reduced levels of H4K20me1 modification.
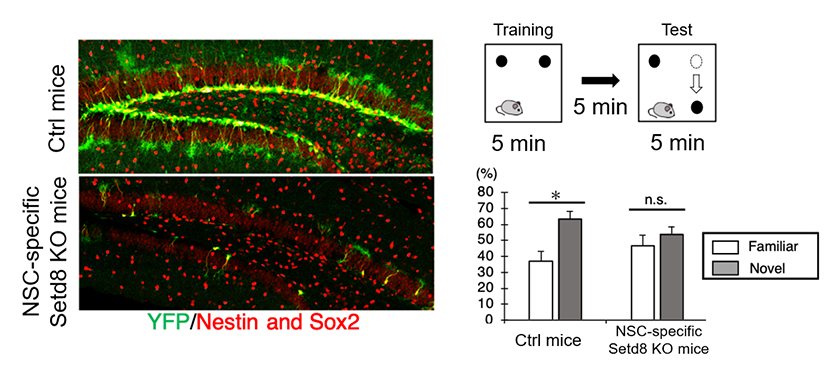
Figure5: Effects of Setd8 deficiency.
In mice with a specific deletion of Setd8 in adult neural stem cells, YFP-positive neural stem cells were reduced (left), resulting in decreased neurogenesis and impaired learning and memory (right). These findings indicate that neural stem cells enter a premature aging state already at a young stage.
Kanae Matsuda NAIST Edge BIO, e0032. (2025).
