RESEARCH
Background: Endoplasmic reticulum (ER) stress and the unfolded protein response (UPR)
The endoplasmic reticulum (ER) is a flat or tubular-shaped sac carried by almost all eukaryotic (animal, plant, and fungous) cells carries. A role of the ER is to fold and modify various proteins, including secretory proteins. Ribosomes attach to the cytosolic side of the ER to form rough ER. Newly synthesized peptides are co-translationally pushed into the sac and transported to the extracellular spaces or other organelles via the vesicular transport system. The ER also serves as a site where lipid molecules are biosynthesized.
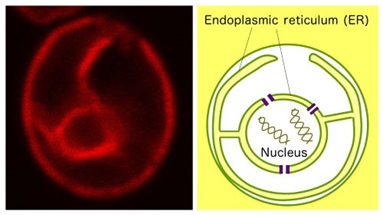
The ER in yeast
S. cerevisiae cells expressing the ER-located protein Elo2 tagged with the red fluorescent protein (RFP) derived from coral.
Dysfunction of the ER is collectively called ER stress and, in many cases, is accompanied with accumulation of aberrant and misfolded client proteins in the ER. For example, overproduction or genetic mutation of an ER client protein causes its aggregation in the ER, triggers ER stress, and damages cells. Some chemicals are also known to induce ER stress.
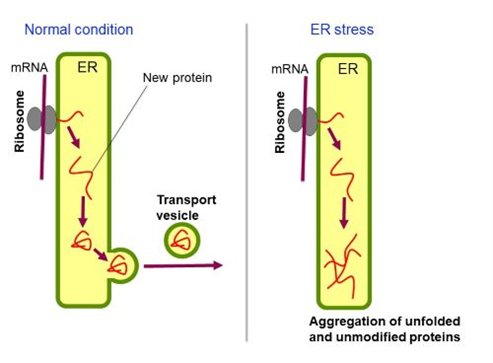
ER stress
Under unstressed normal conditions, new proteins that are pushed into the ER are folded by ER-located molecular chaperones and transported to the cell surface and other organelles via the Golgi apparatus. On the other hand, unfolded proteins accumulate and aggregate in the ER, leading to cellular damage, under ER stress conditions.
Upon ER stress, eukaryotic cells commonly alter their transcriptomes, leading to the enhancement of ER functions. This protective response is known as the unfolded protein response (UPR). While the UPR is commonly conserved in eukaryotic species, its mechanism has been initially uncovered through frontier studies on S. cerevisiae.
Ire1 is an ER-localized transmembrane endoribonuclease. In response to ER stress, Ire1 promotes splicing of the HAC1 mRNA, which is then translated into a nuclear transcription factor Hac1 to trigger the UPR.
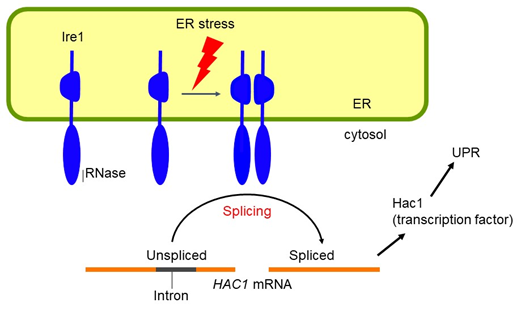
The UPR in S. cerevisaie cells
Under normal unstressed conditions, the HAC1 mRNA remains unspliced and is not translated. In contrast, the spliced HAC1 mRNA produced by Ire1 upon ER stress is translated into the UPR-inducing transcription factor Hac1.
Activation mechanism of Ire1
We found that, in response to ER stress, Ire1 self-associates to form a large oligomeric complex. We have also reported that Ire1 directly detects unfolded proteins that accumulate in the ER. It is likely that ER-accumulated unfolded proteins promote the self-association of Ire1, which then exhibits strong activity to splice the HAC1 mRNA.
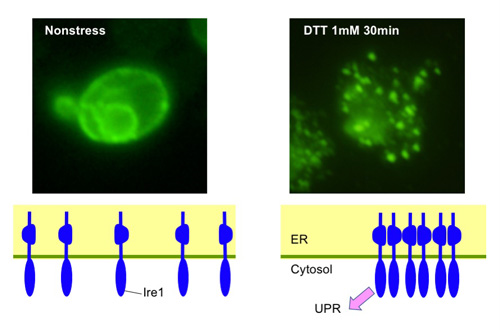
Oligomerization of Ire1 upon ER stress
Ire1 in S. cerevisiae cells was visualized using immunofluorescent technique. While Ire1 is diffusively distributed in non-stressed cells, it shows a dot-like clustered distribution when ER stress is induced by DTT.
On the other hand, Ire1 also triggers the UPR in response to lipid-related abnormality. In this case, Ire1 is activated not via sensing unfolded proteins accumulated in the ER.
ER stress by ethanol
We have searched for conditions under which ER stress is induced using S. cerevisiae as a model organism. Our previous studies revealed that cadmium, which is still a problematic environmental pollutant worldwide, inhibits protein folding in the ER and triggers the UPR. We also observed ER stress and induction of the UPR caused by ethanol.
Although S. cerevisiae is widely used for industrial ethanol fermentation, such as brewing, breadmaking, and bioethanol production, it remains unclear how ER stress and UPR affect this process. To improve the efficiency of ethanol fermentation by yeast, we are focusing on ethanol-induced ER stress.
Physiological change upon UPR
Since Hac1 acts on the promoters of many genes, a wide variety of genes are induced by the UPR. Genes encoding molecular chaperones and protein folding enzymes are predominant targets of Hac1 that is upregulated by the UPR, explaining the role of the UPR to increase the ER function and mitigate ER stress. However, it remains unclear why other genes, such as those encoding sugar transporters and metabolic enzymes, are induced by Hac1 and the UPR. We presume that the UPR may trigger undisclosed physiological changes in S. cerevisiae cells. Using various omics approaches, we will elucidate what happens in yeast cells to mitigate ER stress.
Improved production of biopharmaceuticals and lipidic products from artificially UPR-induced yeast cells
Heterologously expressed human secretory proteins, such as insulin, cytokines, and antibodies, secreted by yeast cells are used as biopharmaceuticals to cure human diseases. Lipidic molecules, such as triglycerides, terpenoids, and lipidic vitamins, which are today obtained from farming plants, will be produced using yeasts in the future. It should be noted that these commercially valuable biomolecules are produced in the ER.
Upon UPR triggered by ER stress, Hac1 transcriptionally induces ER-localized molecular chaperones, protein modification enzymes, and other proteins working for the ER, leading to the enhancement of ER functions. Therefore, it is anticipated that ER function is elevated when an intronless mutant of the HAC1 gene, which is translated into Hac1, is constitutively and unregulatedly expressed. While such cells, namely the constitutively Hac1-expressing cells, are anticipated to carry enforced ER, leading to a high production of commercially valuable biomolecules, one of their disadvantages is that they grow slowly.
Our current mission is to proceed toward application of the constitutively Hac1-expressing cells for the production of valuable biomolecules. To this end, we have obtained fast-growing mutants of the constitutively Hac1-expressing cells. Furthermore, we will demonstrate the high-yield production of biopharmaceuticals and lipidic molecules using the yeast strains created in our study.
For this project, we will use not only S. cerevisiae but also Pichia pastoris, which exhibits high protein secretion.
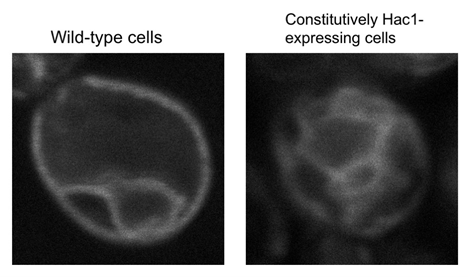
Expansion of the ER in the constitutively Hac1-expressing cells
S. cerevisiae cells producing the ER-marker protein, RFP-labeled Elo2, were observed under a fluorescence microscope.
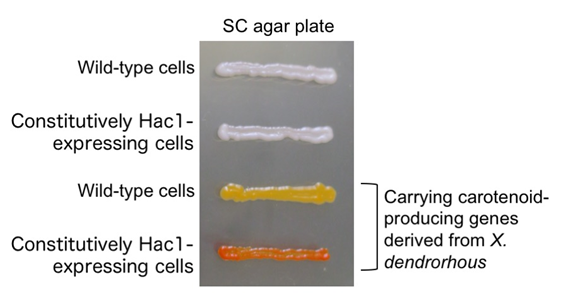
High b-carotene production in the constitutively Hac1-expressing cells
S. cerevisiae cells transformed with a plasmid carrying heterogeneous carotenoid-producing genes or a control empty vector were grown on an agar plate. The constitutively Hac1-expressing cells produced more b-carotene and showed darker red color than wild-type cells.
ER stress induction by human pathogenic proteins in yeast cells
In some human neurodegenerative diseases, such as Parkinson’s and Huntington’s diseases, unfolded or misfolded cytosolic proteins induce ER stress, leading to the aggravation of symptoms. We plan to express cytosolic pathogenic proteins in S. cerevisiae cells and investigate the mechanism by which these proteins damage the ER.